Zinc Oxide (ZnO) and Titanium Dioxide (TiO2) are the only two active ingredients recognised by the EWG and U.S. FDA as posing the lowest hazard of sunscreen ingredients approved by the U.S. FDA and Aus TGA. As discussed in last weeks post, Zinc Oxide is the active ingredient I look for when weighing up a sunscreen’s suitability. But it raises the question, What about nano zinc or nano particles? And are they safe?
After all, the FDA and TGA sunscreen rules allow ANY TYPE of titanium dioxide or zinc oxide to be used in sunscreens and that includes nano titanium dioxide and nano zinc oxide (FDA 2011a) (AS/NZS 2604:2012).
What are Nano and Nano Particles?
Nano refers to the size of the molecules. A nanoparticle is a particle within the nanoscale range of 1 to 100 nanometres in size. A nanometre is one millionth of a millimetre (0.000001 millimetre) and are invisible to the human eye.
The size is important because size determines where the chemical particles can potentially gain access to or how well it can penetrate our skin. Nano particles are extremely small measuring in at 80000 x smaller than the width of the size of a human hair and is able to be absorbed and penetrate more deeply as a result.
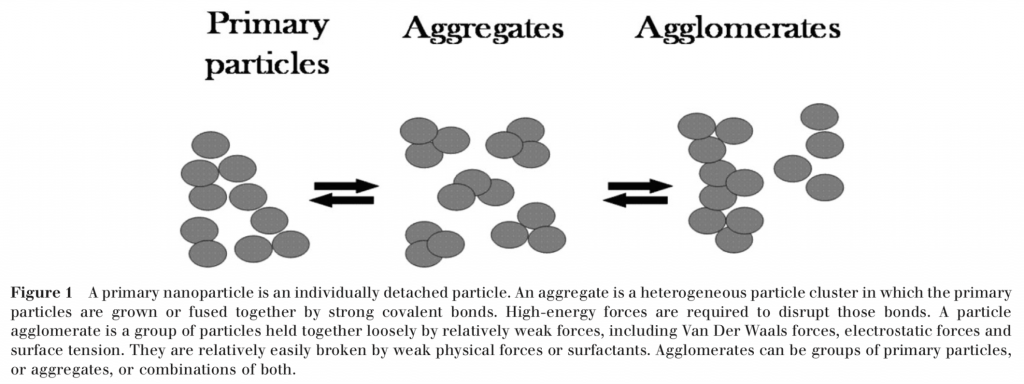
Nanosized TiO2 and ZnO exist in three separate states:
- primary particles (5-20 nm),
- aggregates (30-150 nm), and
- agglomerates (1-100 microns).
Primary particles cluster together to form aggregates and according to studies cited by the TGA they are the smallest units present in a final sunscreen formulation.
Why Are Manufacturers Using Nano Technology?
One reason nano particles have begun to pop up in sunscreen formulas, is because feedback from consumers was that they didn’t like the white/opaque appearance of physical barrier sunscreens on their skin. The size of these barrier oxides were micro sized particles (200-500nm). To reduce the white appearance, manufacturers reduced the size of the particles so they were able to absorb better and were less detectable on the skin.
A nano particles ability to penetrate the skin, reach viable cells and cause harm is what has sparked health concerns.
The reason being is that nano particles of Zinc oxide and titanium dioxide in the presence of UV generates Reactive Oxygen Species which has the ability to damage parts of cells, cause cell death and even cause DNA damage.
To combat this manufacturers generally coat the nano particles with “aluminium hydroxide (Al(OH)3), polymers and inert oxides of silica” or add antioxidants such as vitamins (A, E, C) to stop the formation of Reactive Oxygen Species.
I don’t know about you, but this is a lot of tinkering around just to make some sunscreens appear less white!
Interestingly, the TGA sites a couple of studies that have indicated that our skins natural antioxidant protection adequately combats free radicals generated by nanoparticles all on its own.
So this is all mute if the nano particles don’t actually reach any cells for which they can damage.
What is the TGA’s Stance Based On?
Remember how I mentioned that nano particles can vary in size depending on what state they are in, well the TGA reported that sunscreen formulations use nano particles that are generally relatively large aggregates or even larger agglomerates after the skin application.
After reviewing the scientific literature, the TGA stated that “The majority of in vitro studies (using both animal and human skin) and in vivo studies have shown that both ZnO and TiO2 nano particles either do not penetrate or minimally penetrate the stratum corneum and underlying layers of skin. This suggests that systemic absorption, hence toxicity, is highly unlikely.”
As a result, the TGA concluded that “ZnO or TiO2 nano particles minimally penetrate the underlying layers of skin, with penetration largely limited to the stratum corneum. This suggests that the likelihood of nanoparticles causing cytotoxicity or pathology in internal organs or tissues is very low since systemic absorption is highly unlikely.”
So essentially the government stance is allowing the inclusion of nano particles in sunscreen based of the fact that most studies they reviewed found that nano particles do not penetrate further than the stratum corneum (a surface layer of the epidermis made up of non-viable, keratinized cells in healthy intact skin) and in the case where dermal penetration occurs, the nanoparticles are likely to be adequately counteracted by the defences put in place by manufacturing companies when they coat the particles and/or add antioxidant vitamins.
Other Routes of Exposure
Inhalation
Nano particles suspended in the air pose a great risk for exposure, as the particles are then breathable. Therefore, aerosol sunscreens utilising nano technology pose a unique risk.
Whilst larger micro particles may be pushed out of the body by coughing or sneezing. smaller nano particles have the ability to penetrate the lung. Once in the lungs, the nano particles have the potential to enter the bloodstream and translocate to other organs or cells.
Some studies indicate the ability for nano particles to accumulate in organs such as the heart, brain, liver, spleen, kidneys, and bone marrow. Whilst other studies indicate nano particles can pass through the blood-testis barrier and placental barrier where they accumulate and damage reproductive organs.
Ingestion
I don’t know about you, but I do not anyone who gets ALL the sunscreen off their hands after they have slathered it on their body. This combined with the fact that sunscreens are designed NOT to wash off easily, allows nano particles to be inadvertently consumed when you then eat (and if you are a child who puts their hands in their mouth).
This raises the question, what are the possible adverse health effects of ingested nano particles?
Some studies have raised concerns about ingested nano particles and their effect on the liver, intestinal tissue, spleen and kidney however other studies have not agreed. With the science unclear as to the safety of ingested nano particles, adverse health effects cannot be excluded.
It is all well and good for the TGA to make a judgement using the current data regarding dermal exposure however since it is not the only exposure route for sunscreens containing nano particles AND there are numerous studies citing toxicity when they enter the body, they should reserve their conclusions (and approval of their use) when there is enough scientific studies to confirm safety via all routes of exposure, in my opinion.
Nano particles and Environmental Safety
Human safety aside, it is inevitable that sunscreens enter our environment as they naturally wash off our skin. So what about the environmental impacts of nano particles on aquatic animals and the environment?
Unfortunately, there are no environmental assessments of the nanoparticle’s that enter into the environment. This is concerning as sunscreen ingredients have been shown to accumulate in fish and aquatic animals.
Personally, I would like to see more conclusive evidence before my family reaches for sunscreens that deliberately utilise nano technology. Unfortunately, that is easier said than done.
Non Existent Labelling Laws for Nano Particles in Sunscreen
Labelling laws in the USA, and Australia do not require nano particles to be listed, let alone convey specific size, so comparing one brand against another just got more involved.
Interestingly, the EWG states that even though many companies sell sunscreen specifically advertised as not containing nano zinc or titanium, they believe that the claims are misleading as they believe that nearly all zinc or titanium would be considered nano under the broad FDA definition.
Just to be clear, by law manufacturers ARE NOT required to disclose the qualities of the particles used in their sunscreen so it is a difficult one to authenticate.
Microfine or Micronized Zinc Particles
Some sunscreens state they include “microfine” or “micronized” particles. With more and more negative publicity surrounding the term nano particles, some sunscreen manufacturers are using the term microfine or micronized to describe the zinc oxide used.
But what size are microfine or micronized particles?
Firstly, I would like to point out that there are no regulations or specific definitions for the terms microfine and micronized. So microfine may well be nano sized.
Secondly, even though the Australian Cancer Council states that “microfine particles are larger than nanoparticles and usually range between 100 to 2500 nanometres” a simple search to purchase micronized zinc oxide for sunscreen use, will uncover products with an average particle size of 86 nanometers. Remember <100 is classified as nano sized.
Thirdly, if the Cancer Council are accurate in their size range they state “in the manufacturing process used to produce microfine particles, some particles can inadvertently be ground smaller, ending up being classified as nano-sized. Manufacturers advise this is a small percent and does not classify the sunscreen as nano-based.”
So even if a sunscreen states that it is micronized, if it doesn’t state the AVERAGE particle size and you are trying to avoid nanoparticles, then steer clear of micronized also.
Conclusion
Even though the TGA’s current conclusion states that “the current state of knowledge strongly indicates that the minor risks potentially associated with nanoparticles in sunscreens are vastly outweighed by the benefits that nanoparticle-containing sunscreens afford against skin damage and, importantly, skin cancer,” I disagree.
Firstly, I personally feel the TGA’s conclusion seems to discount the perfectly effective sunscreens on the market that do not utilise the experimental use of nano technology or the other simple methods we have to cover our skin at times it isn’t safe to expose our skin.
Secondly, whether you are pro nano, against or still not sure whether you are comfortable with them in your sunscreens, I think we should be able to make informed purchases based on what we are comfortable with. It is unfortunate that the Australian government doesn’t bring in stronger guidelines as transparent labelling and clear definitions would go a long way to helping us do that.
XxTammy
Want help picking the most protective and non-toxic sunscreen? Click here to jump to last week’s post where I outlined what active ingredients to steer away from and what I look for in a sunscreen.
Want my FREE Guide to Safer Sunscreens where I review 59 different sunscreens? Join my mailing list for your FREE download.
Please Note: The content & media published on this website is the personal opinions of BBMama and are for informational purposes only. It is not intended to be a substitute for professional medical advice and should not be relied on as health or personal advice.
References
Baranowska-Wójcik, E., Szwajgier, D., Oleszczuk, P. and Winiarska-Mieczan, A., 2020. Effects of titanium dioxide nanoparticles exposure on human health—A review. Biological Trace Element Research, 193(1), pp.118-129.
Brand, W., Peters, R.J., Braakhuis, H.M., Maślankiewicz, L. and Oomen, A.G., 2020. Possible effects of titanium dioxide particles on human liver, intestinal tissue, spleen and kidney after oral exposure. Nanotoxicology, 14(7), pp.985-1007.
Butler, M.K., Prow, T.W., Guo, Y.N., Lin, L.L., Webb, R.I. and Martin, D.J., 2012. High-pressure freezing/freeze substitution and transmission electron microscopy for characterization of metal oxide nanoparticles within sunscreens. Nanomedicine, 7(4), pp.541-551.
Cancer Council, 2020. Nanoparticles and sunscreen (online) available: https://www.cancer.org.au/cancer-information/causes-and-prevention/sun-safety/about-sunscreen/nanoparticles-and-sunscreen (last date accessed January 2021)
Osmond-Mcleod MJ, Oytam Y, Kirby JK, Gomez-Fernandez L, Baxter B, McCall MJ. Dermal absorption and short-term biological impact in hairless mice from sunscreens containing zinc oxide nano- or larger particles. Nanotoxicology. 2014;8(SUPPL. 1):72-84.
Osmond-McLeod MJ, Oytam Y, Rowe A, Sobhanmanesh F, Greenoak G, Kirby J, et al. (2016) Long-term exposure to commercially available sunscreens containing nanoparticles of TiO2 and ZnO revealed no biological impact in a hairless mouse model. Particle and fibre toxicology.;13(1):44.
Popov, A.P., Lademann, J., Priezzhev, A.V. and Myllylä, R.A., 2005. Effect of size of TiO 2 nanoparticles embedded into stratum corneum on ultraviolet-A and ultraviolet-B sun-blocking properties of the skin. Journal of biomedical optics, 10(6), p.064037.
Schilling K, Bradford B, Castelli D, Dufour E, Nash JF, Pape W, Schulte S, Tooley I, van den Bosch J & Schellauf F. (2010) Human safety review of “nano” titanium dioxide and zinc oxide. Photochem Photobiol Sci; 9(4): 495-509.
Tran, D.T. and Salmon, R., 2011. Potential photocarcinogenic effects of nanoparticle sunscreens. Australasian journal of dermatology, 52(1), pp.1-6.
Therapeutic Goods Admnistration, 2017. Literature review on the safety of titanium dioxide and zinc oxide on nanoparticles in sunscreens. (online) available: https://www.tga.gov.au/sites/default/files/nanoparticles-sunscreens-review-_2016_1.pdf (last date accessed January 2021)
Wang, Y., Cui, H., Zhou, J., Li, F., Wang, J., Chen, M. and Liu, Q., 2015. Cytotoxicity, DNA damage, and apoptosis induced by titanium dioxide nanoparticles in human non-small cell lung cancer A549 cells. Environmental Science and Pollution Research, 22(7), pp.5519-5530.
Wang, R., Song, B., Wu, J., Zhang, Y., Chen, A. and Shao, L., 2018. Potential adverse effects of nanoparticles on the reproductive system. International Journal of Nanomedicine, 13, p.8487.






My job is to create product labels for sunscreens in Australia and New Zealand. NZ labelling laws do require that nano particles be declared on the label.
Thank you Helen for the clarification.
Unfortunately Australia still continues to keep consumers in the dark in this situation.
The Australian Regulatory Guidelines for Sunscreens issued by the Australian Government’s Dept pf Health states on page 13 under the heading: Nanoparticle ingredients in sunscreens
“The labels of sunscreens are not required to declare the particle sizes of ingredients.”
https://www.tga.gov.au/sites/default/files/australian-regulatory-guidelines-for-sunscreens.pdf